One month after experimental pig heart transplant, doctors say they see no signs of rejection or infection
Originally Published: 20 OCT 23 10:03 ET By Nadia Kounang, CNN
(CNN) — One month after an experimental procedure to transplant the heart of a genetically modified pig into a patient with end-stage heart disease, doctors say the heart is functioning on its own and shows no signs of rejection.
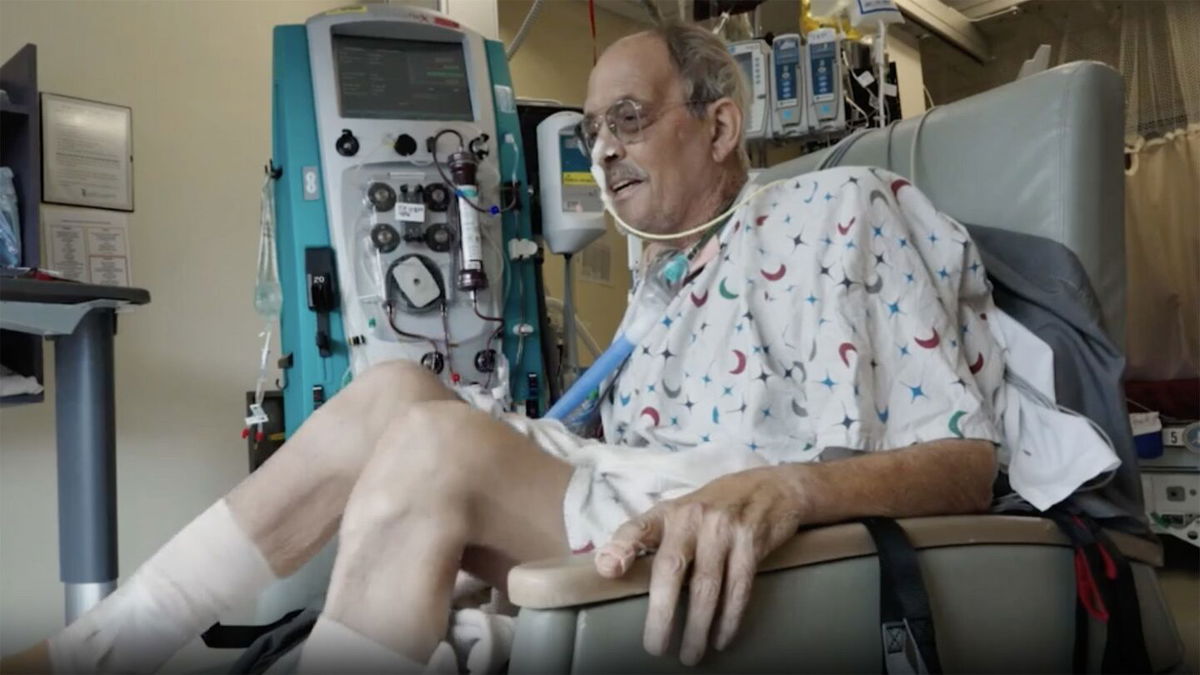
In September, 58-year-old Lawrence Faucette underwent the surgery, only the second ever performed in a human. Faucette’s heart disease and pre-existing conditions made him ineligible for a traditional human heart transplant.
“The physicians taking care of him believe his heart function is excellent,” said Dr. Bartley Griffith, director of the Cardiac and Lung Transplant Program at the University of Maryland School of Medicine who performed the surgery.
“We’ve had no evidence of infections and no evidence of rejection right now.”
Dr. Muhammad Mohiuddin, director of UMMC’s Cardiac Xenotransplantation Program, said in an update shared on Friday, “we are withdrawing all the drugs that were initially supporting his heart. So now his heart is doing everything on its own.”
Mohiuddin said the focus now is making sure that Faucette has the strength to perform routine functions.
“We are working very hard with our physical therapy team who are spending a lot of time helping him regain the strength that he’s lost during last one month of hospital stay,” Mohiuddin said.
In video released by UMMC, Faucette is shown undergoing physical therapy, including cycling to improve his leg strength. When his physical therapist, Chris Wells, reminds him to keep smiling, Faucette laughs and says, “That’s the tough part!”
When Faucette came in, “he never expected, frankly, to be able to stand ever again” said Griffith. While Faucette is not standing on his own yet, he is able to get out of bed with some minimal assistance and doctors say they are at a “pivot point.”
Griffith said that it was time to plan for the next stage of Faucette’s recovery and “thinking about where’s Larry gonna go in terms of his next location.”
Faucette is a married father of two from Frederick, Maryland, and a 20-year Navy veteran who had most recently worked as a lab technician at the National Institutes of Health.
In another moment shared by UMMC, Faucette is seen going over scans of his heart with his doctors. “The one looks like a completely normal heart. And that’s definitely what we wanted,” he says.
Faucette was first admitted to UMMC on September 14 after experiencing symptoms of heart failure. While in the hospital, his heart had stopped twice and was only able to be brought back because of an automatic defibrillator in his room.
“My only real hope left is to go with the pig heart, the xenotransplant,” Faucette told the hospital in an internal interview several days before the surgery.
“We have no expectations other than hoping for more time together,” his wife, Ann Faucette, said at the time. “That could be as simple as sitting on the front porch and having coffee together.”
The experimental xenotransplant surgery was green lit under the US Food and Drug Administration’s “compassionate use” program. According to the FDA, the program is “a potential pathway for a patient with a serious or immediately life-threatening disease or condition to gain access to an investigational medical product (drug, biologic, or medical device) for treatment outside of clinical trials when no comparable or satisfactory alternative therapy options are available.”
The pig heart used came from a genetically modified pig from Revivcor, a subsidiary the United Therapeutics Corporation. The pig had 10 genes edited, including three genes “knocked out” or inactivated to eliminate the alpha gal sugar in the pig’s blood cells, which can trigger a severe reaction in the human immune system, causing organ rejection. An additional pig gene was modified to control for the growth of the pig’s heart while six human genes were added into the pig’s genome to increase acceptance by the immune system. The FDA first approved the gene-edited pigsfor potential therapeutic use and consumption in 2020 .
There are currently no clinical trials that utilize pig organs for transplants in living human beings.
Doctors also treated Faucette with an experimental antibody treatment to further suppress the immune system and prevent rejection. He continues to be monitored for any signs of rejection or any development of pig related viruses. The donor pig was also closely screened for any signs of virus or pathogens.
The hospital said Faucette fully consented to the experimental treatment and was informed of all the risks. In addition, he underwent a full psychiatric evaluation and discussed his case with a medical ethicist.
Mohiuddin and Griffith established the country’s first center for cardiac xenotransplantation research and performed the first such experimental surgery on 57-year-old David Bennett in January 2022. Bennett died two months following the surgery.
While there were no signs of rejection in the initial weeks following the transplant, an autopsy concluded that Bennett ultimately died of heart failure from “a complex array of factors,” including Bennett’s condition prior to the surgery. Bennett had already been hospitalized and kept on a heart lung bypass machine for six weeks prior to the transplant. However, a case study by the doctors published in the Lancet also noted there was evidence of pig virus that had not been identified previously.
According to the federal government, there are more than 113,000 people on the organ transplant list, including more than 3,300 people in need of a heart. The group Donate Life America says that 17 people die each day waiting for a donor organ.
The-CNN-Wire
™ & © 2023 Cable News Network, Inc., a Warner Bros. Discovery Company. All rights reserved.