How soon Covid-19 shots for younger kids could come and what parents need to know now
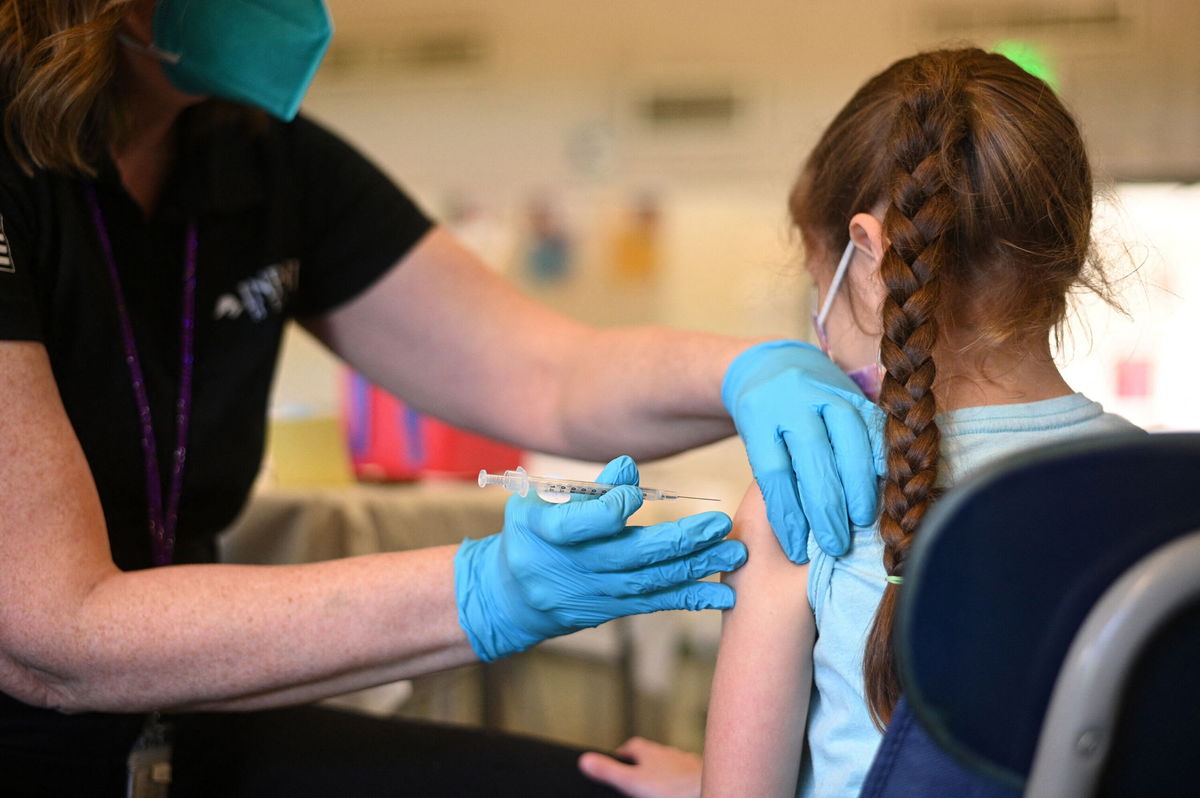
A nurse administers a pediatric dose of the Covid-19 vaccine to a girl in Los Angeles on January 19. Covid-19 vaccines could be authorized for the United States' youngest children as early as June.
By Jacqueline Howard, CNN
Covid-19 vaccines could be authorized for the United States’ youngest children as early as June, according to the US Food and Drug Administration’s latest meeting schedule.
The agency announced Friday it is reserving dates in June for its Vaccines and Related Biological Products Advisory Committee (VRBPAC) to meet to discuss updates to vaccine makers Moderna and Pfizer/BioNTech’s emergency use authorizations that would include making younger ages eligible for Covid-19 vaccination.
Tentative meeting dates for VRBPAC are June 8, 21 and 22.
Following the advisory committee’s deliberations, FDA officials could consider authorizing vaccines for younger children — with that decision also hinging on the agency’s reviews of the vaccine data.
“As we continue to address the ongoing COVID-19 pandemic, there are a number of anticipated submissions and scientific questions that will benefit from discussion with our advisory committee members,” Dr. Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research, said in Friday’s announcement.
“We are providing a tentative schedule for discussion of these submissions, as these meetings will cover a number of topics that are of great interest to the general public,” Marks said. “The agency is committed to a thorough and transparent process that considers the input of our independent advisors and provides insight into our review of the COVID-19 vaccines. We intend to move quickly with any authorizations that are appropriate once our work is completed.”
It has been more than a year since adults first became eligible to receive Covid-19 vaccines in the United States, and currently, children 5 and older are eligible to get vaccinated. But no Covid-19 vaccines have been authorized yet for children younger than 5 in the US — about 18 million people — and, even though FDA advisers plan to meet in June, an exact timeline for potential authorization is still not clear.
Moderna officials have said the FDA is expected to move fast, and a Pfizer official suggested its vaccine for younger children could be available in June, if it’s authorized.
Why is it taking so long to get vaccines for children under 5?
The timeline for making Covid-19 vaccines available for children under 5 hit somewhat of a delay earlier this year when a VRBPAC meeting to discuss the Pfizer/BioNTech vaccine in February was postponed so that additional data on vaccines for this age group could be reviewed.
At the time, the FDA was waiting for Pfizer and BioNTech to submit data from an ongoing trial on a three-dose regimen in these younger children before moving forward with consideration of an emergency use authorization, allowing the FDA to review all data available on the efficacy of each regimen option: two doses or three doses.
If the original meeting had occurred in February, the committee would not have seen all available data to have an informed discussion. The latest clinical trial data from Pfizer/BioNTech suggests that in this age group, two doses seemed to work well against the Delta coronavirus variant but not against the Omicron variant.
As for Moderna’s Covid-19 vaccine, the company announced Thursday that it is seeking emergency use authorization from the FDA for children 6 months through 5 years of age.
Just recently, in late March, Moderna announced results of a clinical trial that included 2,500 children ages 6 months through 24 months and 4,200 children ages 2 through 5. The company said that two 25-microgram doses of its vaccine led to a similar immune response in young children as two 100-microgram doses for adults ages 18 to 25. And it said this should predict protection from Covid-19 and severe Covid-19 down to 6 months of age.
While Moderna has shared some data on two doses of Covid-19 vaccine for younger children, Pfizer and BioNTech’s data on three doses for younger children is not yet available.
The FDA is weighing whether to consider emergency use authorization for both the Pfizer/BioNTech and Moderna Covid-19 vaccines for young children at the same time, rather than considering them separately, Dr. Anthony Fauci, chief medical adviser to President Biden, said last week.
“Two products that are similar but not identical, particularly with regard to the dose, and what the FDA wants to do is to get it so that we don’t confuse people to say ‘this is the dose. This is the dose regimen for children within that age group of 6 months to 5 years,’ ” Fauci, director of the National Institute of Allergy and Infectious Diseases, told CNN’s Kasie Hunt.
The Pfizer vaccine was made to protect against the original strain of the coronavirus, and the initial series of two 3-milligram doses tested in kids under 5 wasn’t powerful enough to keep them safe from the more infectious Omicron variant.
“It didn’t meet the criteria for efficacy,” Fauci said. “There was never a safety issue, but it didn’t meet the criteria, which then had them go back and do a study with a third dose as a part of the primary regimen.”
Pfizer CEO Albert Bourla said publicly that the company’s goal is to have its Covid-19 vaccine available for young children by summer.
How long would it be for my child to get the vaccine after authorization?
If the FDA authorizes the shots, vaccine advisers to the US Centers for Disease Control and Prevention next will vote on whether the vaccine should be recommended for young children. The CDC director will then need to sign off on a vaccine recommendation before shots can be administered.
That process could happen as quickly as within just a few days.
How many kids 5 and older have already received a Covid-19 vaccine?
The youngest group eligible to be vaccinated against Covid-19 in the US, children ages 5 to 11, is also the least vaccinated one, according to the latest CDC data.
Just over a third of 5- to 11-year-olds are fully vaccinated.
But vaccination rates are twice as high among adolescents, and overall, about 43% of currently eligible children ages 5 to 17 are fully vaccinated — about 23 million in all.
Overall, as of Friday, about 66% of the US population is fully vaccinated, including 76% of adults and 90% of seniors. Among adolescents ages 12 to 17, the vaccination rate is 69%.
What’s the risk to kids under 5 if they get Covid-19?
Even though children are far less likely than adults to be hospitalized or to die from Covid-19, it is not a benign disease in young ages, according to the American Academy of Pediatrics (AAP).
The number of new Covid-19 cases among children in the US grew nearly 12% last week from the week before, the AAP announced Monday, but they made up a smaller share of all new cases than they did the week before.
About 37,073 kids tested positive for Covid-19 during the week ending April 21, the second consecutive week of increasing child cases after months of declines.
However, this was 16.3% of all new Covid-19 cases in the US last week, 10 percentage points less than the week before.
Overall, during the Omicron surge, more children were sent to the hospital than in earlier waves. Among states that report hospitalization information by age, kids were 1.2% to 4.6% of the total cumulative hospitalizations, a number that has remained consistent for the past four weeks and has stayed relatively steady throughout the pandemic.
Among states that reported mortality data, kids were up to 0.27% of all Covid-19 deaths, a number that has also remained steady.
For now, how can I protect my child until the Covid-19 vaccine is available?
While parents are waiting to vaccinate their little ones, there is something they can do to protect the children.
All adults who interact with the children should be vaccinated, the experts say, and ideally boosted. Adults are also advised to use masks around unvaccinated children, even as many mandates have fallen away.
Dr. Doran Fink, who oversees the FDA’s clinical and toxicological review of investigational and US-licensed vaccines, said in a meeting of the CDC’s Advisory Committee on Immunization Practices this month that he understood parents’ concerns. He also promised that the FDA would work “diligently” to verify any data submitted.
“We know that many parents and caregivers and health care providers are anxious to have Covid vaccines available for this age group,” Fink said. “I do want to reassure the committee and the public that we understand this concern, and we want to have available safe and effective vaccines for all age groups who will benefit from them.”
The-CNN-Wire
™ & © 2022 Cable News Network, Inc., a WarnerMedia Company. All rights reserved.
CNN’s Brenda Goodman, Jen Christensen and Deidre McPhillips contributed to this report.