FDA approves remdesivir to treat Covid-19
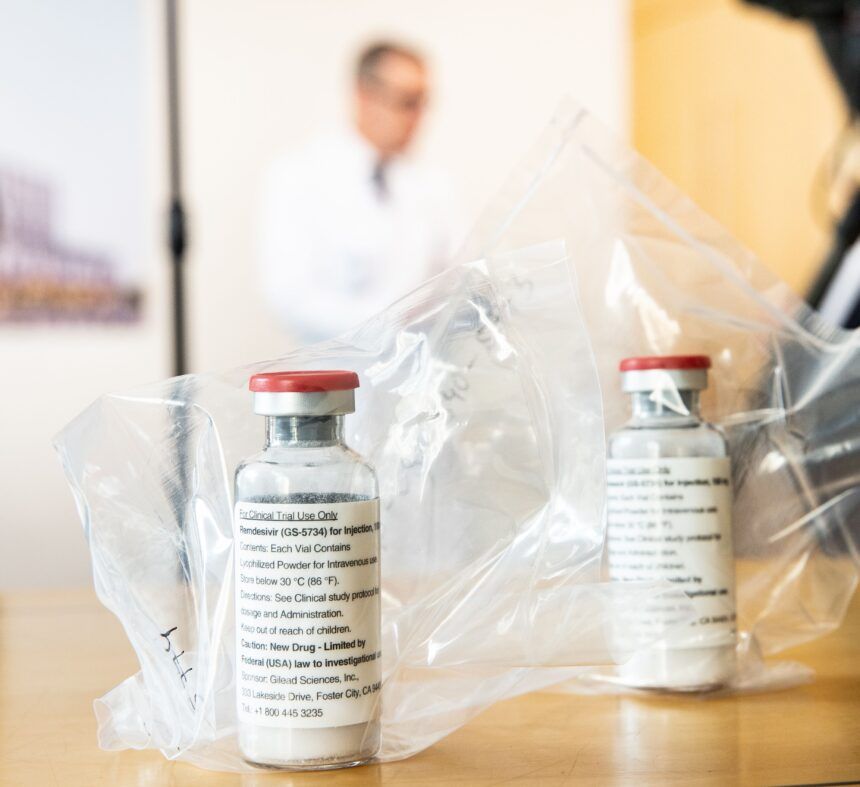
(CNN) The US Food and Drug Administration approved remdesivir for the treatment of coronavirus infection, the drug's maker, Gilead Sciences, said Thursday.
The drug, sold under the brand name Veklury, has been used under emergency use authorization. It is the first drug to be approved for treating Covid-19.
"In the United States, Veklury is indicated for adults and pediatric patients (12 years of age and older and weighing at least 40 kg) for the treatment of COVID-19 requiring hospitalization," the company said in a statement.
"Veklury should only be administered in a hospital or in a healthcare setting capable of providing acute care comparable to inpatient hospital care."
Earlier this month, a World Health Organization-sponsored global study found remdesivir did not help patients survive or even recover faster, but a US study found the infused drug shortened recovery time for some patients by about a third.


